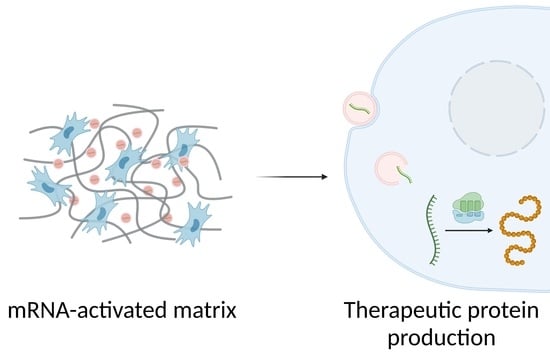
Matrices Activated with Messenger RNA
Abstract
:1. Introduction
2. TAM Design Considerations
2.1. Differences between 2D and 3D Transfection
2.2. Composition and Mechanical Properties of Matrices
2.3. mRNA Modifications
2.4. Nanosystems Employed in mRNA-Activated Matrices
3. Long-Term Stability of mRNA-Activated Matrices
4. Applications
4.1. Bone Regeneration
4.2. Other Regenerative Applications
4.3. Vaccination and Immunomodulation
5. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Langer, R.; Vacanti, J.P. Tissue engineering. Science 1993, 260, 920–926. [Google Scholar] [CrossRef] [Green Version]
- Gaharwar, A.K.; Singh, I.; Khademhosseini, A. Engineered biomaterials for in situ tissue regeneration. Nat. Rev. Mater. 2020, 5, 686–705. [Google Scholar] [CrossRef]
- Griffith, L.G.; Swartz, M.A. Capturing complex 3D tissue physiology in vitro. Nat. Rev. Mol. Cell Biol. 2006, 7, 211–224. [Google Scholar] [CrossRef]
- Huang, G.; Li, F.; Zhao, X.; Ma, Y.; Li, Y.; Lin, M.; Jin, G.; Lu, T.J.; Genin, G.M.; Xu, F. Functional and Biomimetic Materials for Engineering of the Three-Dimensional Cell Microenvironment. Chem. Rev. 2017, 117, 12764–12850. [Google Scholar] [CrossRef] [PubMed]
- Aimin, C.; Chunlin, H.; Juliang, B.; Tinyin, Z.; Zhichao, D. Antibiotic loaded chitosan bar. An in vitro, in vivo study of a possible treatment for osteomyelitis. Clin. Orthop. Relat. Res. 1999, 366, 239–247. [Google Scholar] [CrossRef]
- Arcos, D.; Ragel, C.V.; Vallet-Regí, M. Bioactivity in glass/PMMA composites used as drug delivery system. Biomaterials 2001, 22, 701–708. [Google Scholar] [CrossRef] [PubMed]
- Buckwalter, J.A. Articular cartilage: Injuries and potential for healing. J. Orthop. Sports Phys. Ther. 1998, 28, 192–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weissman, D. mRNA transcript therapy. Expert Rev. Vaccines 2015, 14, 265–281. [Google Scholar] [CrossRef] [PubMed]
- De La Vega, R.E.; van Griensven, M.; Zhang, W.; Coenen, M.J.; Nagelli, C.V.; Panos, J.A.; Peniche Silva, C.J.; Geiger, J.; Plank, C.; Evans, C.H.; et al. Efficient healing of large osseous segmental defects using optimized chemically modified messenger RNA encoding BMP-2. Sci. Adv. 2022, 8, eabl6242. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Zhu, Y.Y.; Smiley, E.; Bonadio, J.; Rouleau, J.P.; Goldstein, S.A.; McCauley, L.K.; Davidson, B.L.; Roessler, B.J. Stimulation of new bone formation by direct transfer of osteogenic plasmid genes. Proc. Natl. Acad. Sci. USA 1996, 93, 5753–5758. [Google Scholar] [CrossRef]
- Wang, C.; Ma, L.; Gao, C. Design of gene-activated matrix for the repair of skin and cartilage. Polym. J. 2014, 46, 476–482. [Google Scholar] [CrossRef]
- Blume, P.; Driver, V.R.; Tallis, A.J.; Kirsner, R.S.; Kroeker, R.; Payne, W.G.; Wali, S.; Marston, W.; Dove, C.; Engler, R.L.; et al. Formulated collagen gel accelerates healing rate immediately after application in patients with diabetic neuropathic foot ulcers. Wound Repair Regen. 2011, 19, 302–308. [Google Scholar] [CrossRef] [Green Version]
- Mulder, G.; Tallis, A.J.; Marshall, V.T.; Mozingo, D.; Phillips, L.; Pierce, G.F.; Chandler, L.A.; Sosnowski, B.K. Treatment of nonhealing diabetic foot ulcers with a platelet-derived growth factor gene-activated matrix (GAM501): Results of a phase 1/2 trial. Wound Repair Regen. 2009, 17, 772–779. [Google Scholar] [CrossRef] [PubMed]
- Leng, Q.; Chen, L.; Lv, Y. RNA-based scaffolds for bone regeneration: Application and mechanisms of mRNA, miRNA and siRNA. Theranostics 2020, 10, 3190–3205. [Google Scholar] [CrossRef]
- Hajj, K.A.; Whitehead, K.A. Tools for translation: Non-viral materials for therapeutic mRNA delivery. Nat. Rev. Mater. 2017, 2, 17056. [Google Scholar] [CrossRef]
- Eskildsen, T.; Taipaleenmäki, H.; Stenvang, J.; Abdallah, B.M.; Ditzel, N.; Nossent, A.Y.; Bak, M.; Kauppinen, S.; Kassem, M. MicroRNA-138 regulates osteogenic differentiation of human stromal (mesenchymal) stem cells in vivo. Proc. Natl. Acad. Sci. USA 2011, 108, 6139–6144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trompeter, H.I.; Dreesen, J.; Hermann, E.; Iwaniuk, K.M.; Hafner, M.; Renwick, N.; Tuschl, T.; Wernet, P. MicroRNAs miR-26a, miR-26b, and miR-29b accelerate osteogenic differentiation of unrestricted somatic stem cells from human cord blood. BMC Genom. 2013, 14, 111. [Google Scholar] [CrossRef] [Green Version]
- Takayama, K.; Suzuki, A.; Manaka, T.; Taguchi, S.; Hashimoto, Y.; Imai, Y.; Wakitani, S.; Takaoka, K. RNA interference for noggin enhances the biological activity of bone morphogenetic proteins in vivo and in vitro. J. Bone Miner. Metab. 2009, 27, 402–411. [Google Scholar] [CrossRef]
- Manaka, T.; Suzuki, A.; Takayama, K.; Imai, Y.; Nakamura, H.; Takaoka, K. Local delivery of siRNA using a biodegradable polymer application to enhance BMP-induced bone formation. Biomaterials 2011, 32, 9642–9648. [Google Scholar] [CrossRef]
- Elangovan, S.; Khorsand, B.; Do, A.V.; Hong, L.; Dewerth, A.; Kormann, M.; Ross, R.D.; Sumner, D.R.; Allamargot, C.; Salem, A.K. Chemically modified RNA activated matrices enhance bone regeneration. J. Control. Release 2015, 218, 22–28. [Google Scholar] [CrossRef]
- Balmayor, E.R.; Geiger, J.P.; Aneja, M.K.; Berezhanskyy, T.; Utzinger, M.; Mykhaylyk, O.; Rudolph, C.; Plank, C. Chemically modified RNA induces osteogenesis of stem cells and human tissue explants as well as accelerates bone healing in rats. Biomaterials 2016, 87, 131–146. [Google Scholar] [CrossRef]
- Ledo, A.M.; Senra, A.; Rilo-Alvarez, H.; Borrajo, E.; Vidal, A.; Alonso, M.J.; Garcia-Fuentes, M. mRNA-activated matrices encoding transcription factors as primers of cell differentiation in tissue engineering. Biomaterials 2020, 247, 120016. [Google Scholar] [CrossRef] [PubMed]
- Leng, Q.; Liang, Z.; Lv, Y. Demineralized bone matrix scaffold modified with mRNA derived from osteogenically pre-differentiated MSCs improves bone repair. Mater. Sci. Eng. C. Mater. Biol. Appl. 2021, 119, 111601. [Google Scholar] [CrossRef] [PubMed]
- Lui, K.O.; Zangi, L.; Silva, E.A.; Bu, L.; Sahara, M.; Li, R.A.; Mooney, D.J.; Chien, K.R. Driving vascular endothelial cell fate of human multipotent Isl1+ heart progenitors with VEGF modified mRNA. Cell Res. 2013, 23, 1172–1186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernal, J.A. RNA-based tools for nuclear reprogramming and lineage-conversion: Towards clinical applications. J. Cardiovasc. Transl. Res. 2013, 6, 956–968. [Google Scholar] [CrossRef] [Green Version]
- Joo, J.Y.; Park, G.Y.; An, S.S. Biocompatible and biodegradable fibrinogen microspheres for tumor-targeted doxorubicin delivery. Int. J. Nanomed. 2015, 10, 101–111. [Google Scholar]
- Yan, J.; Chen, R.; Zhang, H.; Bryers, J.D. Injectable Biodegradable Chitosan-Alginate 3D Porous Gel Scaffold for mRNA Vaccine Delivery. Macromol. Biosci. 2019, 19, e1800242. [Google Scholar] [CrossRef]
- Chen, R.; Zhang, H.; Yan, J.; Bryers, J.D. Scaffold-mediated delivery for non-viral mRNA vaccines. Gene Ther. 2018, 25, 556–567. [Google Scholar] [CrossRef]
- Dastmalchi, F.; Karachi, A.; Mehkri, Y.; O’Malley, A.; Subramaniam, V.; Angelini, T.; Mitchell, D.; Rahman, M. IMMU-20. HYDROGEL-CXCL9 VACCINE RESULTS IN MRNA DELIVERY TO DENDRITIC CELLS AND POTENT ANTI-TUMOR RESPONSES IN GBM. Neuro Oncol. 2021, 23, vi96. [Google Scholar] [CrossRef]
- Dhaliwal, A.; Oshita, V.; Segura, T. Transfection in the third dimension. Integr. Biol. 2013, 5, 1206–1216. [Google Scholar] [CrossRef] [Green Version]
- Badieyan, Z.S.; Berezhanskyy, T.; Utzinger, M.; Aneja, M.K.; Emrich, D.; Erben, R.; Schüler, C.; Altpeter, P.; Ferizi, M.; Hasenpusch, G.; et al. Transcript-activated collagen matrix as sustained mRNA delivery system for bone regeneration. J. Control. Release 2016, 239, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Balmayor, E.R.; Geiger, J.P.; Koch, C.; Aneja, M.K.; van Griensven, M.; Rudolph, C.; Plank, C. Modified mRNA for BMP-2 in Combination with Biomaterials Serves as a Transcript-Activated Matrix for Effectively Inducing Osteogenic Pathways in Stem Cells. Stem Cells Dev. 2017, 26, 25–34. [Google Scholar] [CrossRef]
- Zaitseva, T.S.; Yang, G.; Dionyssiou, D.; Zamani, M.; Sawamura, S.; Yakubov, E.; Ferguson, J.; Hallett, R.L.; Fleischmann, D.; Paukshto, M.V.; et al. Delivery of hepatocyte growth factor mRNA from nanofibrillar scaffolds in a pig model of peripheral arterial disease. Regen. Med. 2020, 15, 1761–1773. [Google Scholar] [CrossRef] [PubMed]
- Fayed, O.; van Griensven, M.; Birgani, Z.T.; Plank, C.; Balmayor, E.R. Transcript-Activated Coatings on Titanium Mediate Cellular Osteogenesis for Enhanced Osteointegration. Mol. Pharm. 2021, 18, 1121–1137. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, A.; Kormann, M.; Rosenecker, J.; Rudolph, C. Current prospects for mRNA gene delivery. Eur. J. Pharm. Biopharm. 2009, 71, 484–489. [Google Scholar] [CrossRef] [PubMed]
- Steinle, H.; Ionescu, T.M.; Schenk, S.; Golombek, S.; Kunnakattu, S.J.; Özbek, M.T.; Schlensak, C.; Wendel, H.P.; Avci-Adali, M. Incorporation of Synthetic mRNA in Injectable Chitosan-Alginate Hybrid Hydrogels for Local and Sustained Expression of Exogenous Proteins in Cells. Int. J. Mol. Sci. 2018, 19, 1313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, P.; Perche, F.; Midoux, P.; Cabral, C.S.D.; Malard, V.; Correia, I.J.; EI-Hafci, H.; Petite, H.; Logeart-Avramoglou, D.; Pichon, C. In Vivo bone tissue induction by freeze-dried collagen-nanohydroxyapatite matrix loaded with BMP2/NS1 mRNAs lipopolyplexes. J. Control. Release 2021, 334, 188–200. [Google Scholar] [CrossRef]
- Schneider-Barthold, C.; Baganz, S.; Wilhelmi, M.; Scheper, T.; Pepelanova, I. Hydrogels based on collagen and fibrin-Frontiers and applications. BioNanoMaterials 2016, 17, 3–12. [Google Scholar] [CrossRef]
- Patel, S.; Athirasala, A.; Menezes, P.P.; Ashwanikumar, N.; Zou, T.; Sahay, G.; Bertassoni, L.E. Messenger RNA Delivery for Tissue Engineering and Regenerative Medicine Applications. Tissue Eng. Part A 2019, 25, 91–112. [Google Scholar] [CrossRef]
- Kundu, J.; Pati, F.; Shim, J.H.; Cho, D.W. 10-Rapid prototyping technology for bone regeneration. In Rapid Prototyping of Biomaterials; Narayan, R., Ed.; Woodhead Publishing: Sawston, UK, 2014; pp. 254–284. [Google Scholar]
- Hesse, E.; Hefferan, T.E.; Tarara, J.E.; Haasper, C.; Meller, R.; Krettek, C.; Lu, L.; Yaszemski, M.J. Collagen type I hydrogel allows migration, proliferation, and osteogenic differentiation of rat bone marrow stromal cells. J. Biomed. Mater. Res. A 2010, 94, 442–449. [Google Scholar] [CrossRef] [Green Version]
- Almelkar, S.I.; Patwardhan, A.M.; Divate, S.A.; Agrawal, N.B.; Bhonde, R.R.; Chaukar, A.P. Fibrin matrix supports endothelial cell adhesion and migration in culture. OA Biol. 2014, 2, 5. [Google Scholar]
- Zhang, W.; De La Vega, R.E.; Coenen, M.J.; Müller, S.A.; Peniche Silva, C.J.; Aneja, M.K.; Plank, C.; van Griensven, M.; Evans, C.H.; Balmayor, E.R. An Improved, Chemically Modified RNA Encoding BMP-2 Enhances Osteogenesis In Vitro and In Vivo. Tissue Eng. Part A 2019, 25, 131–144. [Google Scholar] [CrossRef] [PubMed]
- Oude Egberink, R.; Zegelaar, H.M.; El Boujnouni, N.; Versteeg, E.M.M.; Daamen, W.F.; Brock, R. Biomaterial-Mediated Protein Expression Induced by Peptide-mRNA Nanoparticles Embedded in Lyophilized Collagen Scaffolds. Pharmaceutics 2022, 14, 1619. [Google Scholar] [CrossRef] [PubMed]
- Geng, Y.; Duan, H.; Xu, L.; Witman, N.; Yan, B.; Yu, Z.; Wang, H.; Tan, Y.; Lin, L.; Li, D.; et al. BMP-2 and VEGF-A modRNAs in collagen scaffold synergistically drive bone repair through osteogenic and angiogenic pathways. Commun. Biol. 2021, 4, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Khorsand, B.; Elangovan, S.; Hong, L.; Dewerth, A.; Kormann, M.S.D.; Salem, A.K. A Comparative Study of the Bone Regenerative Effect of Chemically Modified RNA Encoding BMP-2 or BMP-9. AAPS J. 2017, 19, 438–446. [Google Scholar] [CrossRef] [PubMed]
- Khorsand, B.; Elangovan, S.; Hong, L.; Kormann, M.S.D.; Salem, A.K. A bioactive collagen membrane that enhances bone regeneration. J. Biomed. Mater. Res. B. Appl. Biomater. 2019, 107, 1824–1832. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Chen, T.; Song, Y.; Feng, C.; Chen, H.; Zhang, Q.; Chen, G.; Zhu, X. mRNA Delivery by a pH-Responsive DNA Nano-Hydrogel. Small 2021, 17, e2101224. [Google Scholar] [CrossRef]
- Zaitseva, T.S.; Alcazar, C.; Zamani, M.; Hou, L.; Sawamura, S.; Yakubov, E.; Hopkins, M.; Woo, Y.J.; Paukshto, M.V.; Huang, N.F. Aligned Nanofibrillar Scaffolds for Controlled Delivery of Modified mRNA. Tissue Eng. Part A 2019, 25, 121–130. [Google Scholar] [CrossRef]
- Houchin-Ray, T.; Swift, L.A.; Jang, J.; Shea, L.D. Patterned PLG substrates for localized DNA delivery and directed neurite extension. Biomaterials 2007, 28, 2603–2611. [Google Scholar] [CrossRef] [Green Version]
- Huang, N.F.; Patel, S.; Thakar, R.G.; Wu, J.; Hsiao, B.S.; Chu, B.; Lee, R.J.; Li, S. Myotube assembly on nanofibrous and micropatterned polymers. Nano Lett. 2006, 6, 537–542. [Google Scholar] [CrossRef]
- Downing, T.L.; Soto, J.; Morez, C.; Houssin, T.; Fritz, A.; Yuan, F.; Chu, J.; Patel, S.; Schaffer, D.V.; Li, S. Biophysical regulation of epigenetic state and cell reprogramming. Nat. Mater. 2013, 12, 1154–1162. [Google Scholar] [CrossRef]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef]
- Haugh, M.G.; Murphy, C.M.; McKiernan, R.C.; Altenbuchner, C.; O’Brien, F.J. Crosslinking and Mechanical Properties Significantly Influence Cell Attachment, Proliferation, and Migration Within Collagen Glycosaminoglycan Scaffolds. Tissue Eng. Part A 2011, 17, 1201–1208. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Liu, M.; Chen, G.; Xu, Z.; Lv, Y. Demineralized Bone Scaffolds with Tunable Matrix Stiffness for Efficient Bone Integration. ACS Appl. Mater. Interfaces 2018, 10, 27669–27680. [Google Scholar] [CrossRef] [PubMed]
- Keeney, M.; Onyiah, S.; Zhang, Z.; Tong, X.; Han, L.H.; Yang, F. Modulating polymer chemistry to enhance non-viral gene delivery inside hydrogels with tunable matrix stiffness. Biomaterials 2013, 34, 9657–9665. [Google Scholar] [CrossRef] [PubMed]
- Ledo, A.M.; Vining, K.H.; Alonso, M.J.; Garcia-Fuentes, M.; Mooney, D.J. Extracellular matrix mechanics regulate transfection and SOX9-directed differentiation of mesenchymal stem cells. Acta Biomater. 2020, 110, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix Elasticity Directs Stem Cell Lineage Specification. Cell 2006, 126, 677–689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hennink, W.E.; van Nostrum, C.F. Novel crosslinking methods to design hydrogels. Adv. Drug Deliv. Rev. 2012, 64, 223–236. [Google Scholar] [CrossRef]
- Williams, J.K.; Yoo, J.J.; Atala, A. Chapter 59-Regenerative Medicine Approaches for Tissue Engineered Heart Valves. In Principles of Regenerative Medicine, 3rd ed.; Atala, A., Lanza, R., Mikos, A.G., Nerem, R., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 1041–1058. [Google Scholar]
- Jeon, O.; Ryu, S.H.; Chung, J.H.; Kim, B.S. Control of basic fibroblast growth factor release from fibrin gel with heparin and concentrations of fibrinogen and thrombin. J. Control. Release 2005, 105, 249–259. [Google Scholar] [CrossRef]
- Gandhi, J.K.; Knudsen, T.; Hill, M.; Roy, B.; Bachman, L.; Pfannkoch-Andrews, C.; Schmidt, K.N.; Metko, M.M.; Ackerman, M.J.; Resch, Z.; et al. Human Fibrinogen for Maintenance and Differentiation of Induced Pluripotent Stem Cells in Two Dimensions and Three Dimensions. Stem Cells Transl. Med. 2019, 8, 512–521. [Google Scholar] [CrossRef] [Green Version]
- Horasawa, N.; Yamashita, T.; Uehara, S.; Udagawa, N. High-performance scaffolds on titanium surfaces: Osteoblast differentiation and mineralization promoted by a globular fibrinogen layer through cell-autonomous BMP signaling. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 46, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Rahim, M.; Ng, Q.; Segura, T. Hyaluronic acid and fibrin hydrogels with concentrated DNA/PEI polyplexes for local gene delivery. J. Control. Release 2011, 153, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Lutolf, M.P.; Lauer-Fields, J.; Schmoekel, H.G.; Metters, A.T.; Weber, F.E.; Fields, G.B.; Hubbell, J.A. Synthetic matrix metalloproteinase-sensitive hydrogels for the conduction of tissue regeneration: Engineering cell-invasion characteristics. Proc. Natl. Acad. Sci. USA 2003, 100, 5413–5418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Utzinger, M.; Jarzebinska, A.; Haag, N.; Schweizer, M.; Winter, G.; Dohmen, C.; Rudolph, C.; Plank, C. cmRNA/lipoplex encapsulation in PLGA microspheres enables transfection via calcium phosphate cement (CPC)/PLGA composites. J. Control. Release 2017, 249, 143–149. [Google Scholar] [CrossRef]
- Sanz-Horta, R.; Matesanz, A.; Jorcano, J.L.; Velasco, D.; Acedo, P.; Gallardo, A.; Reinecke, H.; Elvira, C. Preparation and Characterization of Plasma-Derived Fibrin Hydrogels Modified by Alginate di-Aldehyde. Int. J. Mol. Sci. 2022, 23, 4296. [Google Scholar] [CrossRef]
- Martina, M.; Hutmacher, D.W. Biodegradable polymers applied in tissue engineering research: A review. Polym. Int. 2007, 56, 145–157. [Google Scholar] [CrossRef]
- Arun, Y.; Ghosh, R.; Domb, A.J. Biodegradable Hydrophobic Injectable Polymers for Drug Delivery and Regenerative Medicine. Adv. Funct. Mater. 2021, 31, 2010284. [Google Scholar] [CrossRef]
- Khalil, A.S.; Yu, X.; Umhoefer, J.M.; Chamberlain, C.S.; Wildenauer, L.A.; Diarra, G.M.; Hacker, T.A.; Murphy, W.L. Single-dose mRNA therapy via biomaterial-mediated sequestration of overexpressed proteins. Sci. Adv. 2020, 6, eaba2422. [Google Scholar] [CrossRef]
- Jemielity, J.; Fowler, T.; Zuberek, J.; Stepinski, J.; Lewdorowicz, M.; Niedzwiecka, A.; Stolarski, R.; Darzynkiewicz, E.; Rhoads, R.E. Novel “anti-reverse” cap analogs with superior translational properties. RNA 2003, 9, 1108–1122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leppek, K.; Das, R.; Barna, M. Functional 5’ UTR mRNA structures in eukaryotic translation regulation and how to find them. Nat. Rev. Mol. Cell Biol. 2018, 19, 158–174. [Google Scholar] [CrossRef] [Green Version]
- Holtkamp, S.; Kreiter, S.; Selmi, A.; Simon, P.; Koslowski, M.; Huber, C.; Türeci, O.; Sahin, U. Modification of antigen-encoding RNA increases stability, translational efficacy, and T-cell stimulatory capacity of dendritic cells. Blood 2006, 108, 4009–4017. [Google Scholar] [CrossRef] [PubMed]
- Karikó, K.; Buckstein, M.; Ni, H.; Weissman, D. Suppression of RNA recognition by Toll-like receptors: The impact of nucleoside modification and the evolutionary origin of RNA. Immunity 2005, 23, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Kormann, M.S.D.; Hasenpusch, G.; Aneja, M.K.; Nica, G.; Flemmer, A.W.; Herber-Jonat, S.; Huppmann, M.; Mays, L.E.; Illenyi, M.; Schams, A.; et al. Expression of therapeutic proteins after delivery of chemically modified mRNA in mice. Nat. Biotechnol. 2011, 29, 154–157. [Google Scholar] [CrossRef] [PubMed]
- Warren, L.; Manos, P.D.; Ahfeldt, T.; Loh, Y.; Li, H.; Lau, F.; Ebina, W.; Mandal, P.K.; Smith, Z.D.; Meissner, A.; et al. Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell 2010, 7, 618–630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, S.; Tang, X.; Chen, Y.; Chen, K.; Fan, N.; Xiao, W.; Zheng, Q.; Li, G.; Teng, Y.; Wu, M.; et al. mRNA-based therapeutics: Powerful and versatile tools to combat diseases. Signal Transduct. Target Ther. 2022, 7, 166. [Google Scholar] [CrossRef]
- Uchida, S.; Itaka, K.; Chen, Q.; Osada, K.; Ishii, T.; Shibata, M.; Harada-Shiba, M.; Kataoka, K. PEGylated polyplex with optimized PEG shielding enhances gene introduction in lungs by minimizing inflammatory responses. Mol. Ther. 2012, 20, 1196–1203. [Google Scholar] [CrossRef] [Green Version]
- Godbey, W.T.; Wu, K.K.; Mikos, A.G. Poly(ethylenimine) and its role in gene delivery. J. Control. Release 1999, 60, 149–160. [Google Scholar] [CrossRef]
- Bettinger, T.; Carlisle, R.C.; Read, M.L.; Ogris, M.; Seymour, L.W. Peptide-mediated RNA delivery: A novel approach for enhanced transfection of primary and post-mitotic cells. Nucleic Acids Res. 2001, 29, 3882–3891. [Google Scholar] [CrossRef] [Green Version]
- Elangovan, S.; D’Mello, S.R.; Hong, L.; Ross, R.D.; Allamargot, C.; Dawson, D.V.; Stanford, C.M.; Johnson, G.K.; Sumner, D.R.; Salem, A.K. The enhancement of bone regeneration by gene activated matrix encoding for platelet derived growth factor. Biomaterials 2014, 35, 737–747. [Google Scholar] [CrossRef] [Green Version]
- Ferkol, T.; Perales, J.C.; Eckman, E.; Kaetzel, C.S.; Hanson, R.W.; Davis, P.B. Gene transfer into the airway epithelium of animals by targeting the polymeric immunoglobulin receptor. J. Clin. Investig. 1995, 95, 493–502. [Google Scholar] [CrossRef] [Green Version]
- Wagner, E.; Cotten, M.; Foisner, R.; Birnstiel, M.L. Transferrin-polycation-DNA complexes: The effect of polycations on the structure of the complex and DNA delivery to cells. Proc. Natl. Acad. Sci. USA 1991, 88, 4255–4259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, B.; Zhang, S.; Jiang, H.; Zhao, B.; Lv, H. Lipoplex morphologies and their influences on transfection efficiency in gene delivery. J. Control. Release 2007, 123, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Ishida, T.; Okada, Y.; Oku, N.; Kiwada, H. Increased gene expression by cationic liposomes (TFL-3) in lung metastases following intravenous injection. Biol. Pharm. Bull. 2005, 28, 701–706. [Google Scholar] [CrossRef] [Green Version]
- Persano, S.; Guevara, M.L.; Li, Z.; Mai, J.; Ferrari, M.; Pompa, P.P.; Shen, H. Lipopolyplex potentiates anti-tumor immunity of mRNA-based vaccination. Biomaterials 2017, 125, 81–89. [Google Scholar] [CrossRef] [Green Version]
- Su, X.; Fricke, J.; Kavanagh, D.G.; Irvine, D.J. In vitro and in vivo mRNA delivery using lipid-enveloped pH-responsive polymer nanoparticles. Mol. Pharm. 2011, 8, 774–787. [Google Scholar] [CrossRef] [Green Version]
- Mével, M.; Neveu, C.; Gonçalves, C.; Yaouanc, J.; Pichon, C.; Jaffrès, P.; Midoux, P. Novel neutral imidazole-lipophosphoramides for transfection assays. Chem. Commun. 2008, 27, 3124–3126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.; Han, D.; Cai, C.; Tang, X. An overview of liposome lyophilization and its future potential. J. Control. Release 2010, 142, 299–311. [Google Scholar] [CrossRef]
- Mesquida, P.; Kohl, D.; Andriotis, O.G.; Thurner, P.J.; Duer, M.; Bansode, S.; Schitter, G. Evaluation of surface charge shift of collagen fibrils exposed to glutaraldehyde. Sci. Rep. 2018, 8, 10126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reckhenrich, A.K.; Hopfner, U.; Krötz, F.; Zhang, Z.; Koch, C.; Kremer, M.; Machens, H.-G.; Plank, C.; Egaña, J.T. Bioactivation of dermal scaffolds with a non-viral copolymerprotected gene vector. Biomaterials 2011, 32, 1996–2003. [Google Scholar] [CrossRef]
- Scherer, F.; Schillinger, U.; Putz, U.; Stemberger, A.; Plank, C. Nonviral vector loaded collagen sponges for sustained gene delivery in vitro and in vivo. J. Gene Med. 2002, 4, 634–643. [Google Scholar] [CrossRef]
- Zhang, L.; Glasser, F.P. Critical examination of drying damage to cement pastes. Adv. Cem. Res. 2000, 12, 79–88. [Google Scholar] [CrossRef]
- Xu, D.; Zhao, Y.; Wang, J.; He, J.; Weng, Y.; Luo, J. Smads, p38 and ERK1/2 are involved in BMP9-induced osteogenic differentiation of C3H10T1/2 mesenchymal stem cells. BMB Rep. 2012, 45, 247–252. [Google Scholar] [CrossRef]
- Karam, M.; Daoud, G. mRNA vaccines: Past, present, future. Asian J. Pharm. Sci. 2022, 17, 491–522. [Google Scholar] [CrossRef] [PubMed]
- McNamara, M.A.; Nair, S.K.; Holl, E.K. RNA-Based Vaccines in Cancer Immunotherapy. J. Immunol. Res. 2015, 2015, 794528. [Google Scholar] [CrossRef] [Green Version]
- Fotin-Mleczek, M.; Duchardt, K.M.; Lorenz, C.; Pfeiffer, R.; Ojkić-Zrna, S.; Probst, J.; Kallen, K.J. Messenger RNA-based vaccines with dual activity induce balanced TLR-7 dependent adaptive immune responses and provide antitumor activity. J. Immunother. 2011, 34, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Peng, K.; Qiu, L.; Li, M.; Ruan, J.; He, L.; Yuan, Z. Hitchhiking on Controlled-Release Drug Delivery Systems: Opportunities and Challenges for Cancer Vaccines. Front. Pharmacol. 2021, 12, 679602. [Google Scholar] [CrossRef] [PubMed]
- Stachowiak, A.N.; Irvine, D.J. Inverse opal hydrogel-collagen composite scaffolds as a supportive microenvironment for immune cell migration. J. Biomed. Mater. Res. A 2008, 85, 815–828. [Google Scholar] [CrossRef]




| Application | Matrix Composition | Encoded Protein | mRNA Modifications | Transfection Reagents | Development | Publication Year |
|---|---|---|---|---|---|---|
| Bone regeneration | Collagen scaffold | BMP-2 | ARCA | PEI | In vitro and in vivo (rat critical-sized calvarial bone defect model) | 2015 [20] |
| s2U (0.25) m5C (0.25) or Ψ (1.0) m5C (1.0) | ||||||
| Poly(A) - 120 | ||||||
| Bone regeneration | Collagen scaffold | BMP-7 | Cap 1 structure | PepFect14 or Lipofectamine™ MessengerMAX | In vitro | 2022 [44] |
| Sequence modifications | ||||||
| Bone regeneration | Collagen scaffold | BMP-2 or BMP-9 | ARCA | PEI | In vitro and in vivo (rat critical-sized calvarial defect model) | 2017 [46] |
| Ψ (1.0) m5C (1.0) | ||||||
| Poly(A) - 120 | ||||||
| Bone regeneration | Collagen fibre matrix | BMP-2 and VEGF-A | m1Ψ (1.0) | Lipofectamine™ MessengerMAX | In vitro and in vivo (rat critical-sized calvarial defect model) | 2021 [45] |
| Poly(A) tail | ||||||
| Bone regeneration | Collagen-nanohydroxyapatite matrix | BMP-2 and NS1 | Poly(A) tail | Lipopolyplexes His-lPEI/Lip100 or Lipofectamine™ MessengerMax | In vitro and in vivo (mouse ectopic model) | 2021 [37] |
| Bone regeneration | Collagen sponge scaffold | BMP-2 | Cap structure | Proprietary lipid/DPPC/cholesterol/DMG-PEG or PEI | In vitro and in vivo (rat non-critical femoral bone defect model) | 2016 [31] |
| s2U m5C | ||||||
| Poly(A) - 200 | ||||||
| Bone healing | Collagen sponge scaffold | BMP-2 | TISU sequence | Proprietary lipid/DPPC/cholesterol/DMG-PEG | In vivo (rat critical-sized femoral osteotomies defect) | 2022 [9] |
| 5IU (0.35) 5IC (0.075) | ||||||
| Bone healing | Collagen sponge scaffold | BMP-2 | ARCA | Proprietary lipid/DPPC/cholesterol/DMG-PEG | In vitro and in vivo (rat critical-sized femoral defect) | 2019 [43] |
| TISU sequence | ||||||
| 5IU (0.35) 5IC (0.075) or s2U (0.25) m5C (0.25) | ||||||
| Poly(A) - 120 | ||||||
| Poly(A) tail | ||||||
| Bone regeneration | Perforated collagen membranes | BMP-9 | ARCA | PEI | In vitro and in vivo (rat critical-sized calvarial defect model) | 2019 [47] |
| Ψ (1.0) m5C (1.0) | ||||||
| Poly(A) - 120 | ||||||
| Bone regeneration | Demineralized bone matrix scaffold | Oi-mRNA | None | PEI | In vitro and in vivo (rat critical-sized calvarial defect model) | 2021 [23] |
| Bone healing | Fibrin gel | BMP-2 | ARCA | Proprietary lipid/DOPE/cholesterol/DMPE-PEG | In vitro and in vivo (non-critical rat femur bone defect model) | 2016 [21] |
| s2U (0.25) m5C (0.25) | ||||||
| Bone regeneration | Fibrin gel or micro-macro biphasic calcium phosphate (MBCP) ceramic granules | BMP-2 | ARCA | DreamFect™ Gold | In vitro | 2017 [32] |
| s2U (0.25) m5C (0.25) | ||||||
| Poly(A) tail | ||||||
| Bone healing | PLGA microspheres in calcium phosphate cements | Reporter proteins | s2U m5C | Proprietary lipid/DOPE/cholesterol/DMG-PEG | In vitro | 2017 [66] |
| Poly(A) - 200 | ||||||
| Ortho-regeneration | Poly-D,L-lactic acid (PDLLA), fibrin or fibrinogen coating | BMP-2 | ARCA | Proprietary lipid/DPPC/cholesterol/DMG-PEG | In vitro | 2021 [34] |
| 5IU (0.35) 5IC (0.075) | ||||||
| Poly(A) - 200 | ||||||
| Tissue engineering | Chitosan-alginate hybrid hydrogels | Reporter proteins | ARCA | GenaxxoFect™ reagent | In vitro | 2018 [36] |
| Ψ (1.0) m5C (1.0) | ||||||
| mRNA delivery | DNA nano-hydrogel | Reporter proteins | m7G cap | None | In vitro | 2021 [48] |
| Poly (A) tail | ||||||
| Chondrogenesis and myogenesis | Fibrin gel | SOX-9 or MYOD | ARCA | 3DfectIN™ | In vitro | 2020 [22] |
| Kozak consensus sequence | ||||||
| alpha-globin 3′ UTR terminating | ||||||
| Vascular regeneration | Matrigel™ | VEGF-A | ARCA | Lipofectamine™ RNAiMAX | In vitro and in vivo (NOD/SCID mice) | 2013 [24] |
| Ψ m5C | ||||||
| Poly(A) tail | ||||||
| Vascular regeneration | Parallel-aligned nanofibrillar collagen scaffolds | HGF | Cap 1 structure | Lipofectamine™ Messenger Max | In vitro and in vivo (porcine peripheral arterial disease model) | 2020 [33] |
| Ψ m5C | ||||||
| Poly(A) - 175 | ||||||
| Wound healing | Mineral-coated microparticles (MCMs) | bFGF | ARCA | Lipofectamine™ Messenger Max | In vitro and in vivo (murine model of diabetic ulcers) | 2020 [70] |
| Ψ m5C | ||||||
| Poly(A) tail | ||||||
| Vaccine | pHEMA scaffold | Reporter proteins | m7G cap | Lipofectamine™ Messenger Max or Stemfect™ or in vivo-jetPEI™ or Poly (β-amino ester)) | In vitro and in vivo (mouse subcutaneous implant model) | 2018 [28] |
| Poly(A) tail | ||||||
| Vaccine | Chitosan-alginate 3D porous gel | OVA | m7G cap | Stemfect™ | In vitro and in vivo (murine model) | 2018 [27] |
| poly(A) tail | ||||||
| Vaccine | Hydrogel | Tumour proteins | None | Nanoparticles | In vitro and in vivo (murine glioblastoma multiforme model) | 2021 [29] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martinez-Campelo, R.; Garcia-Fuentes, M. Matrices Activated with Messenger RNA. J. Funct. Biomater. 2023, 14, 48. https://doi.org/10.3390/jfb14010048
Martinez-Campelo R, Garcia-Fuentes M. Matrices Activated with Messenger RNA. Journal of Functional Biomaterials. 2023; 14(1):48. https://doi.org/10.3390/jfb14010048
Chicago/Turabian StyleMartinez-Campelo, Raquel, and Marcos Garcia-Fuentes. 2023. "Matrices Activated with Messenger RNA" Journal of Functional Biomaterials 14, no. 1: 48. https://doi.org/10.3390/jfb14010048